Numerous tests – in vivo and in vitro, have been performed on the biocompatibility and bioeffectiveness of Epifibroin Powder.
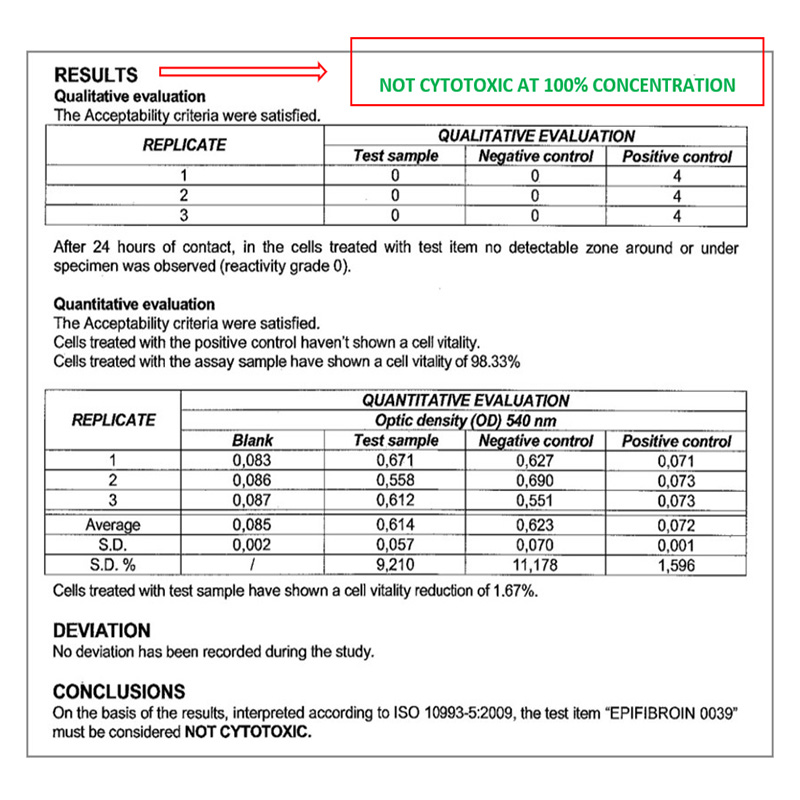
Biocompatibility – In vitro test
CYTOTOXICITY TEST BY DIRECT CONTACT
Conducted at Eurofins Biolab S.r.l., Eurofins Scientific Group, Milan – Italy according to ISO 10993-5: 2009 – (Good Laboratory Practice)
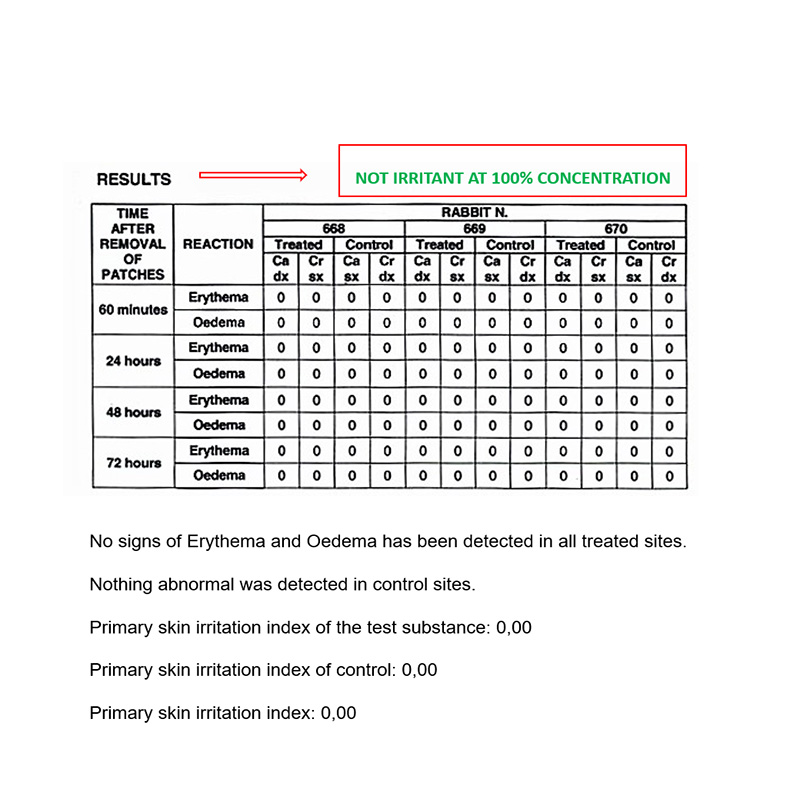
Biocompatibility – In vivo test
IN VIVO CUTANEOUS IRRITATION TEST
Conducted at Eurofins Biolab S.r.l., Eurofins Scientific Group, Milan – Italy according to ISO 10993-5: 2009 – (Good Laboratory Practice)
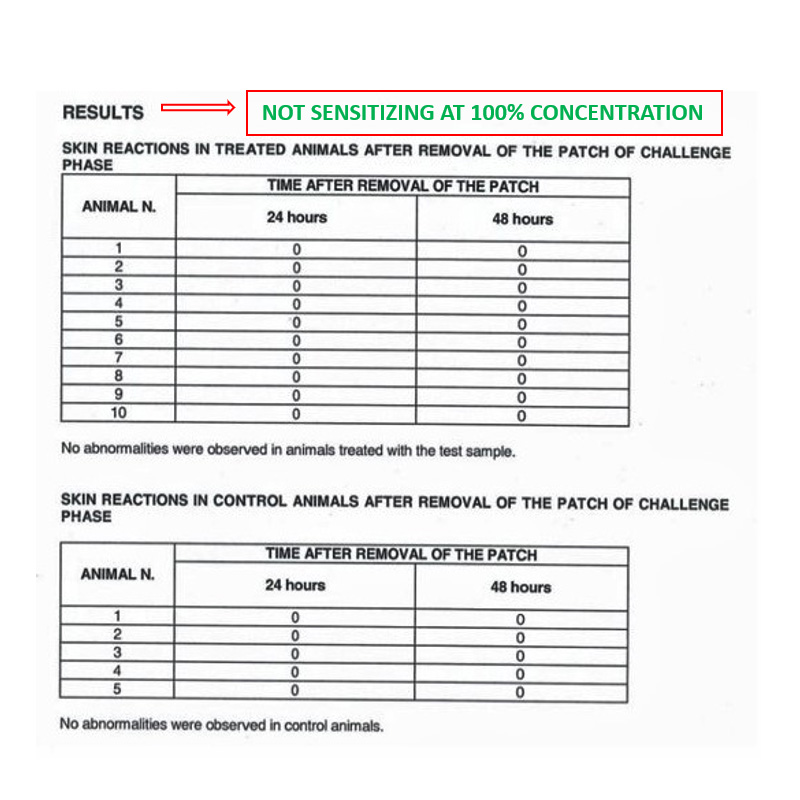
Biocompatibility – In vivo test
IN VIVO DELAYED HYPERSENSITIVITY TEST
Conducted at Eurofins Biolab S.r.l., Eurofins Scientific Group, Milan – Italy according to ISO 10993-5: 2009 – (Good Laboratory Practice)
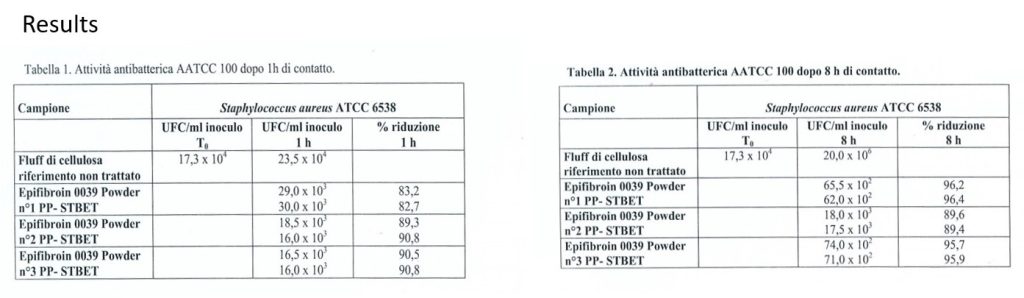
Bioeffectiveness – In vitro test
Analysis: AATCC 100/98 quantitative antibacterial activity
Microorganism: Staphylococcus aureus ATCC 6538
Contact time: 1 and 8 hours at 37 ° C
Test report No. 1643 – 10.12.2014